LIFA vTau SPAD-powered FLIM Camera
LIFA vTau Fluorescence Lifetime Imaging Microscopy, in a nutshell
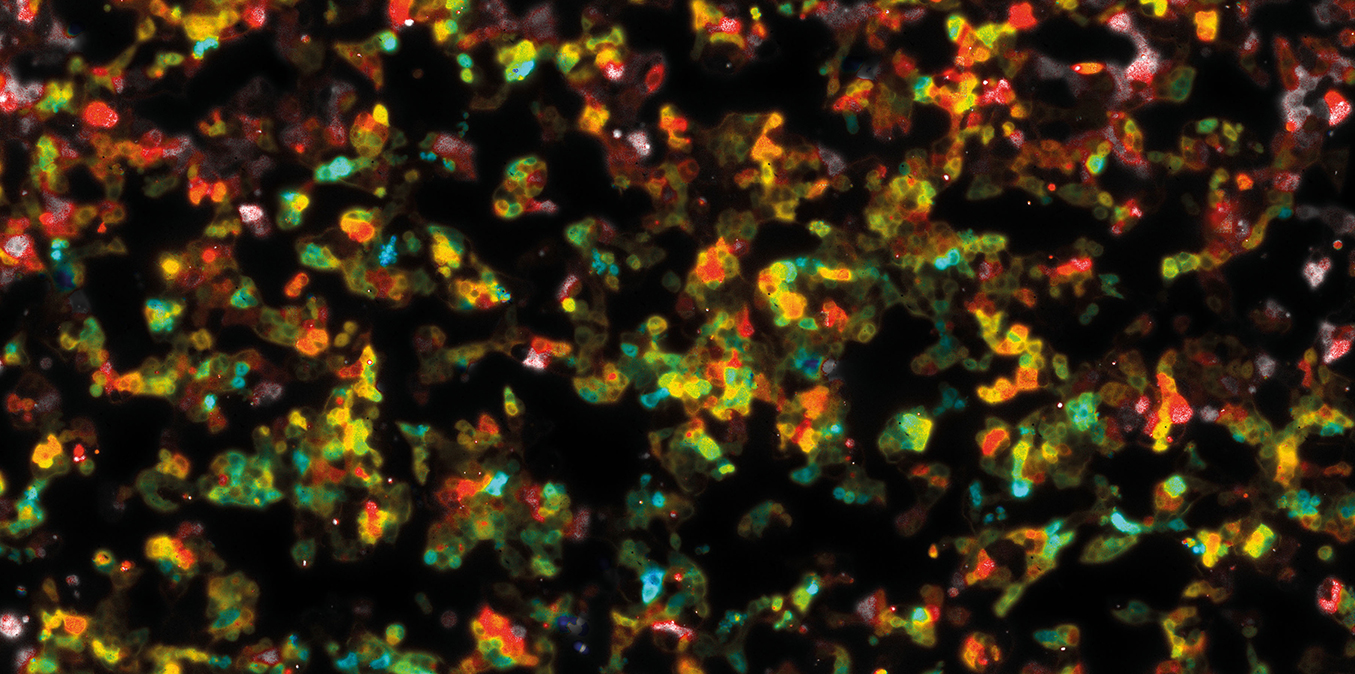
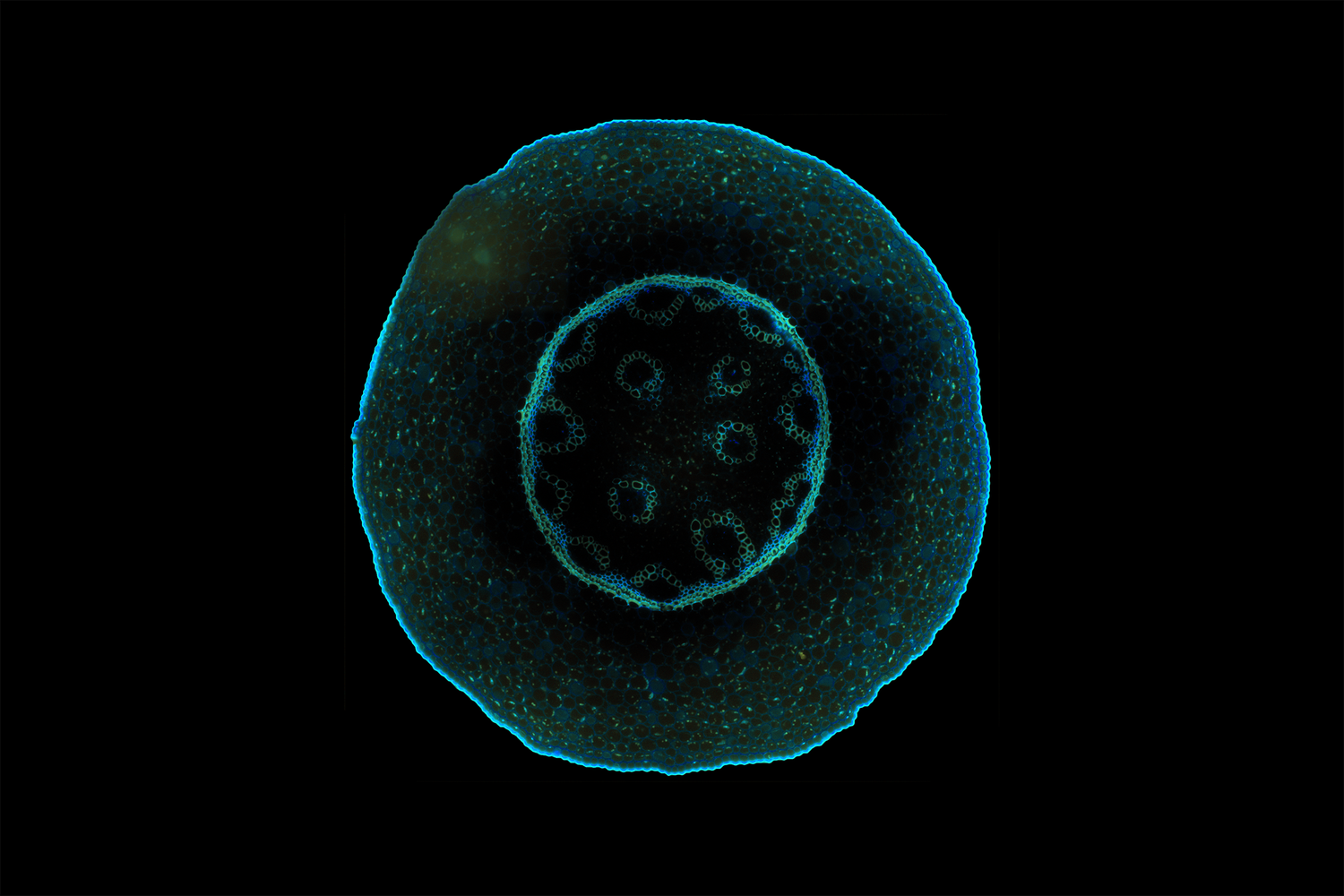
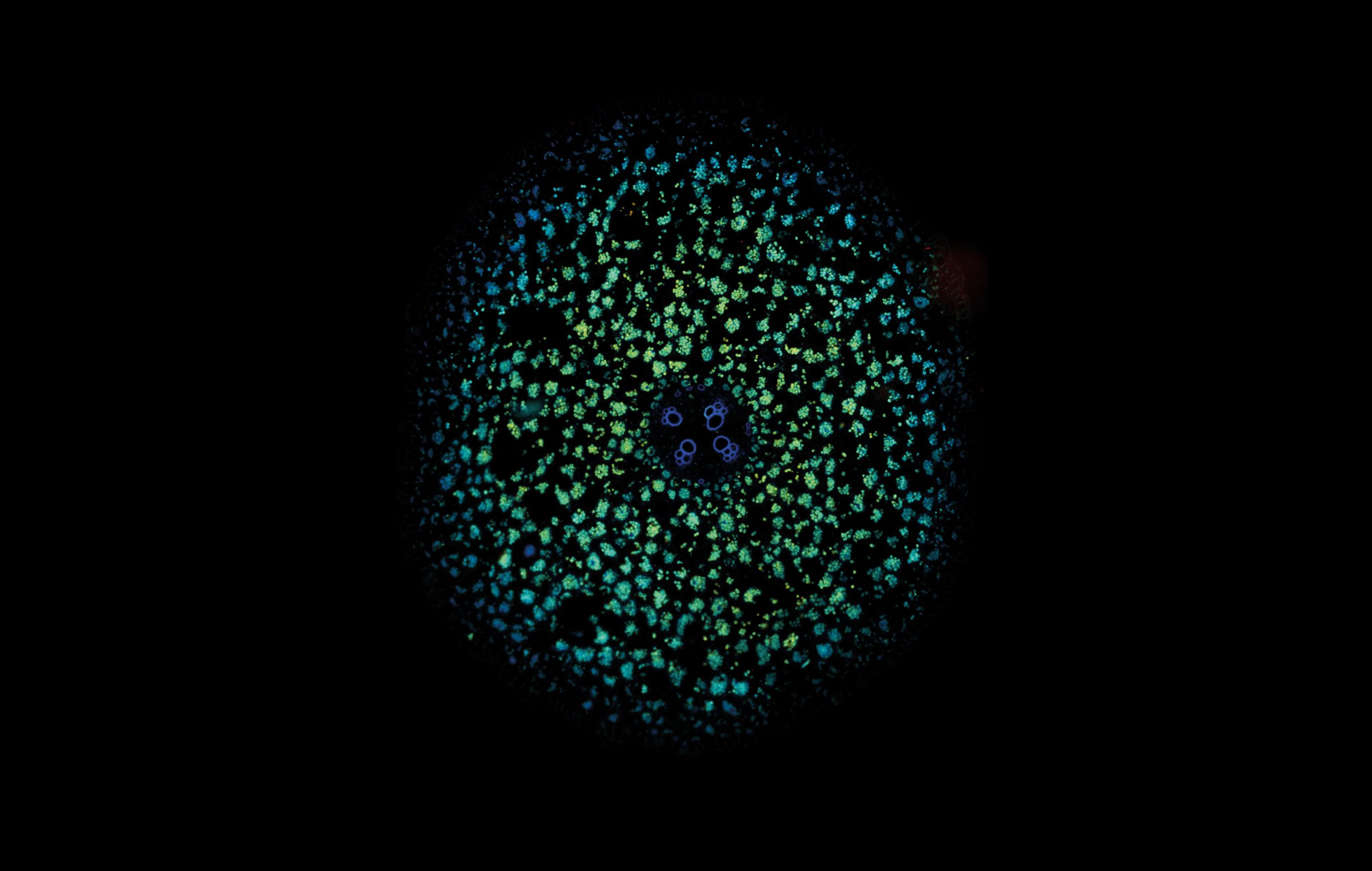
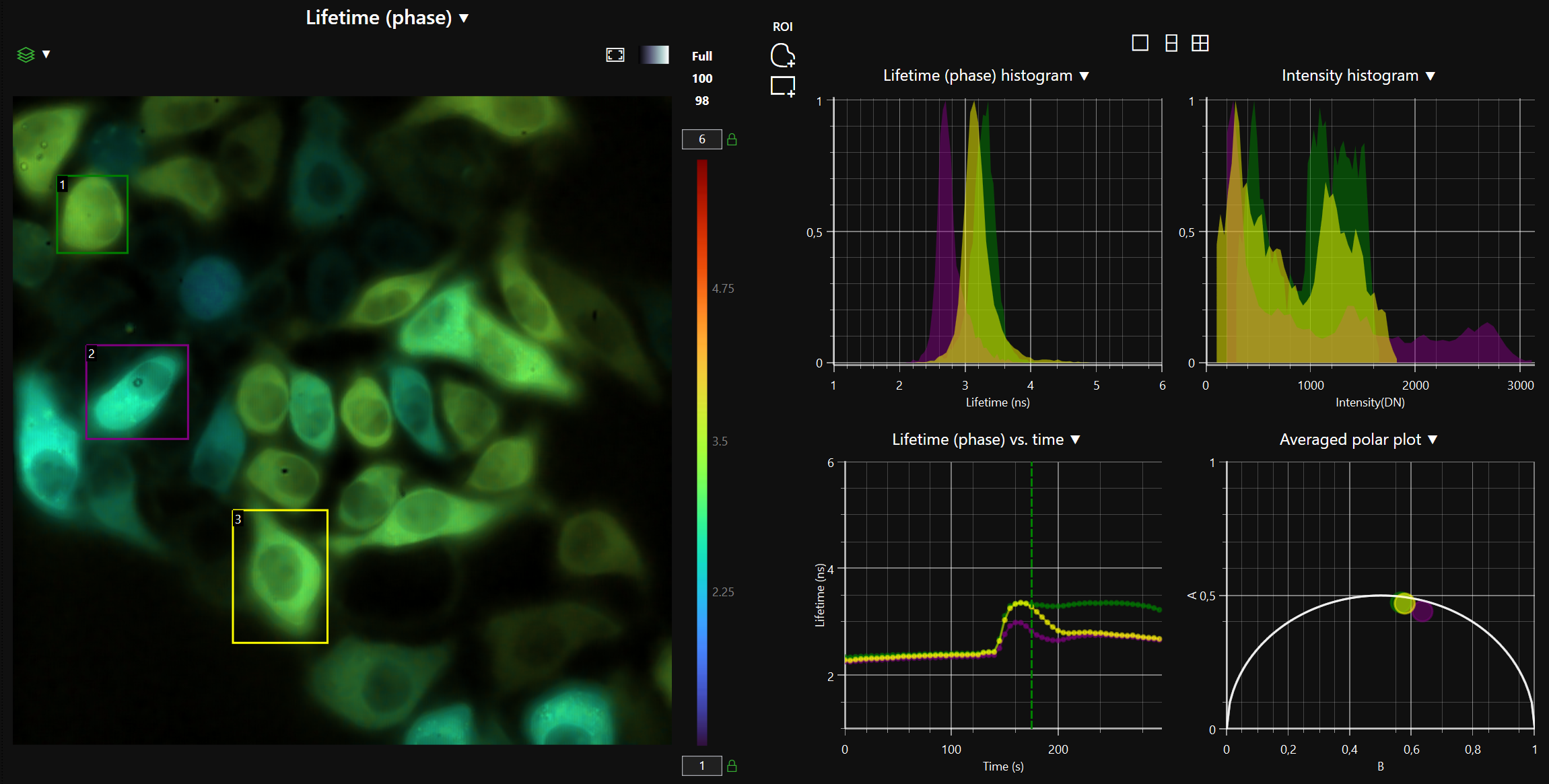
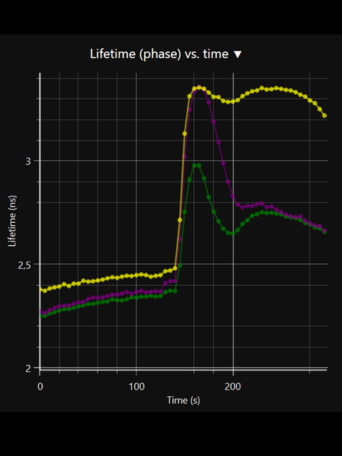
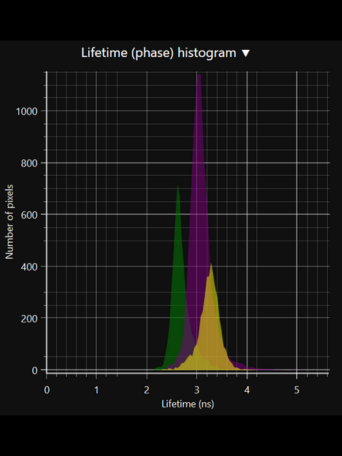
LIFA vTAU is a camera-based system for fast fluorescence lifetime imaging microscopy (FLIM), especially well-suited for live cell applications. Featuring vTAU, our versatile SPAD camera, the system allows for near instantaneous acquisition of lifetime images at unprecedented frame rates with high accuracy. The Lambert Instruments LIFA vTau is the fastest and easiest way to do fluorescence lifetime imaging microscopy (FLIM).
Available in versatile configurations dependent on your specific applications, the LIFA vTau system offers a turn-key solution for fluorescence lifetime imaging microscopy. Compatible with every fluorescence microscope with a camera output – including microscopes by Leica, Nikon, Olympus, and Zeiss; set up is easy and fast, with all hardware integrating seamlessly with our LIFA software, so you can focus on your experiment.
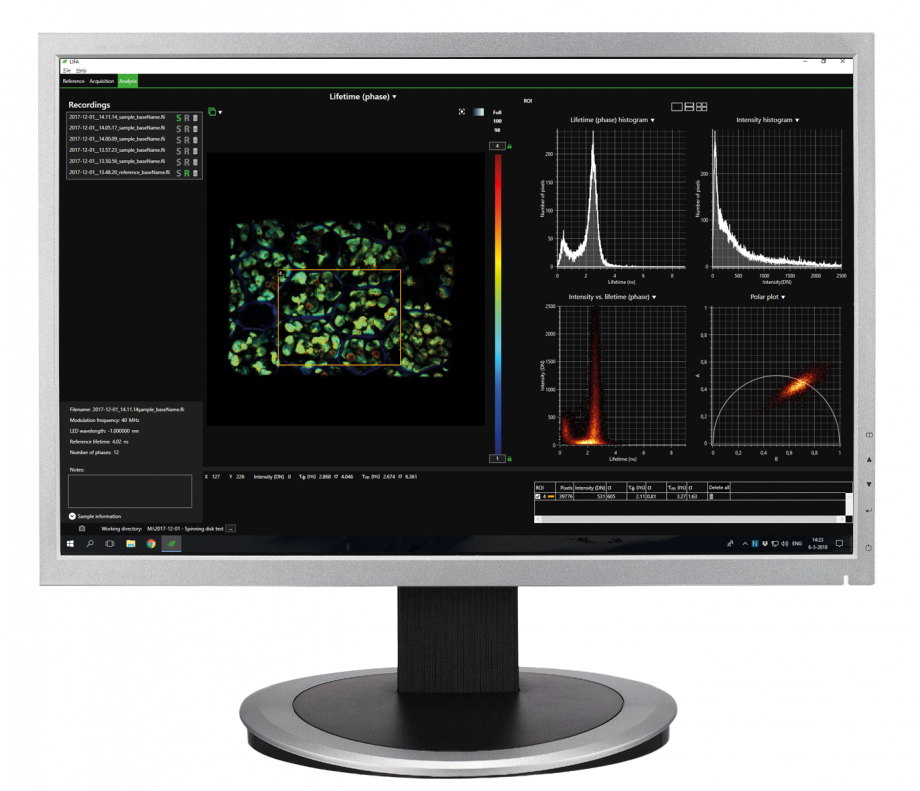
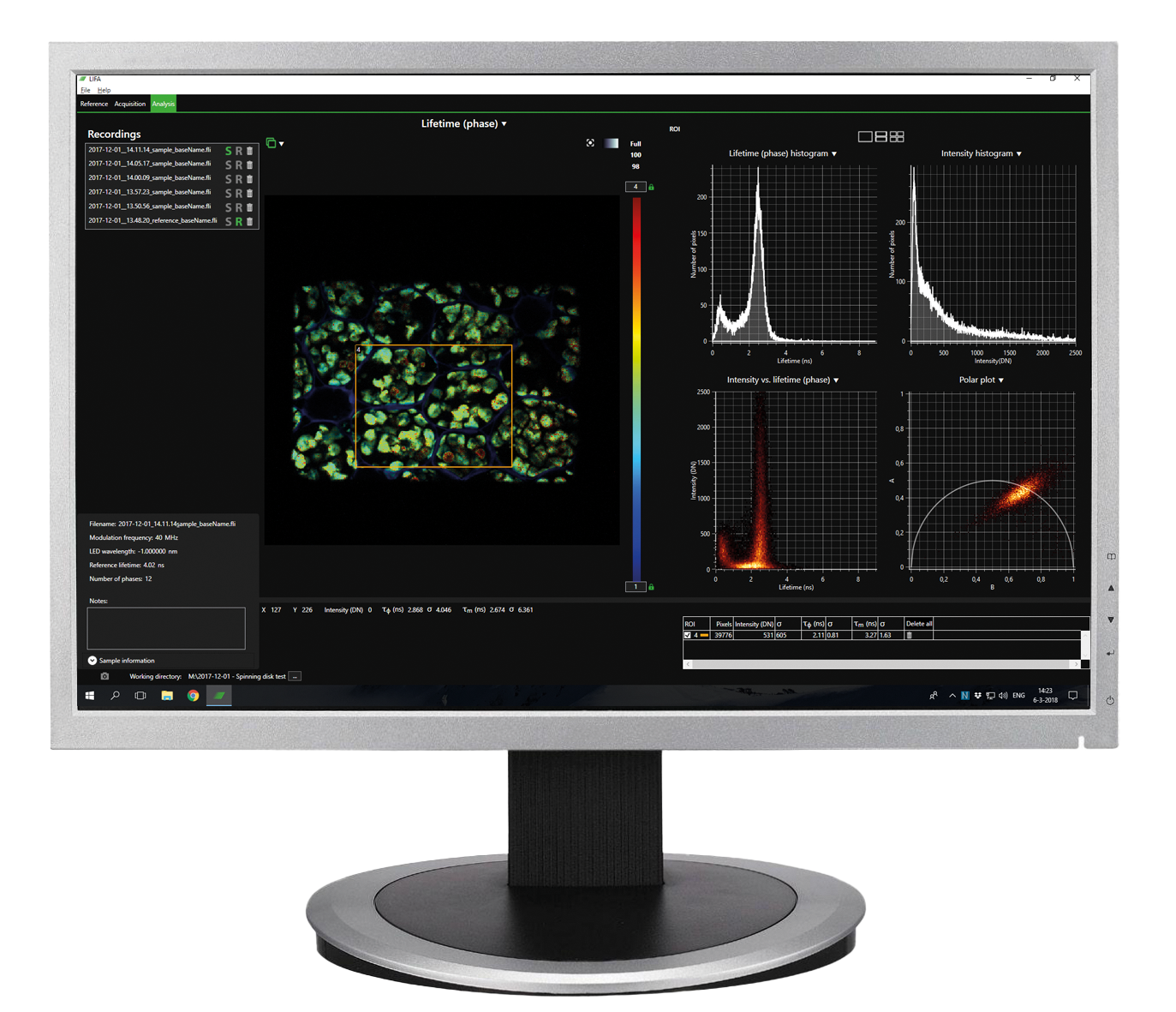
The advanced software instantly analyzes your data and presents the calculated fluorescence lifetimes visually. Recorded images are compatible with ImageJ, FIJI, MatLab and MetaMorph, while detailed statistical data can be exported to Excel worksheets.
LIFA vTau Fluorescence Lifetime Imaging Microscopy, in more details
Simplify experiments for researchers and imaging centers with the new vTAU SPAD camera; combining excellent light sensitivity with easy image acquisition and data analysis.
Minimize measurement duration, automate image acquisition, and simplify data analysis… factors of great importance for cell biology, cancer research and high-throughput screening.
LIFA vTAU easily integrates into any FLIM system, providing a plug-and-play experience that allows for switching between setups.
Key features & specifications
- Complete solution: LIFA vTAU works with any brand of fluorescence microscope to form a fully integrated FLIM system for a complete solution from sample to data.
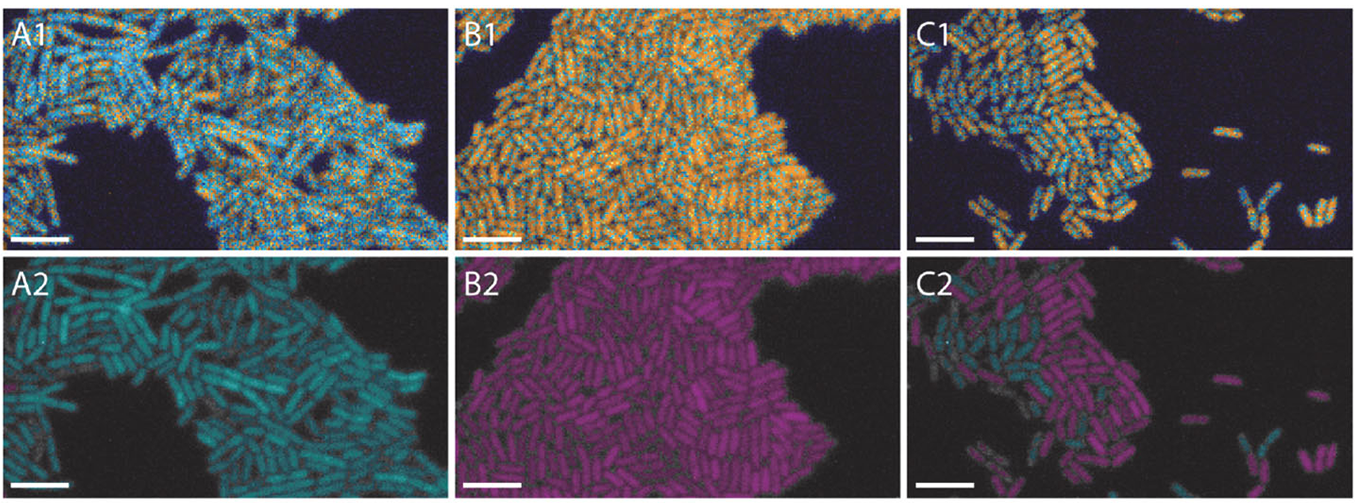
- Multiple configurations: Suitable for widefield, spinning-disk confocal, light-sheet, and TIRF modalities, LIFA vTAU delivers an easy plug-and-play setup experience.
- Fast, adaptive dynamic range: Utilizing the latest ultra-high sensitivity SPAD detector, featuring micro-lenses to improve light efficiency and using ultra-short exposures to optimize dynamic range, vTAU can capture up to 100 lifetime images per second in challenging light conditions.
- Next level sensitivity: With the unique properties of SPAD, vTAU features excellent light sensitivity to minimize measurement duration, and enables noise-free readout of the detector.
- Broad lifetime range: Matching a wide range of capabilities, vTAU operates from the sub microsecond down to picoseconds range.
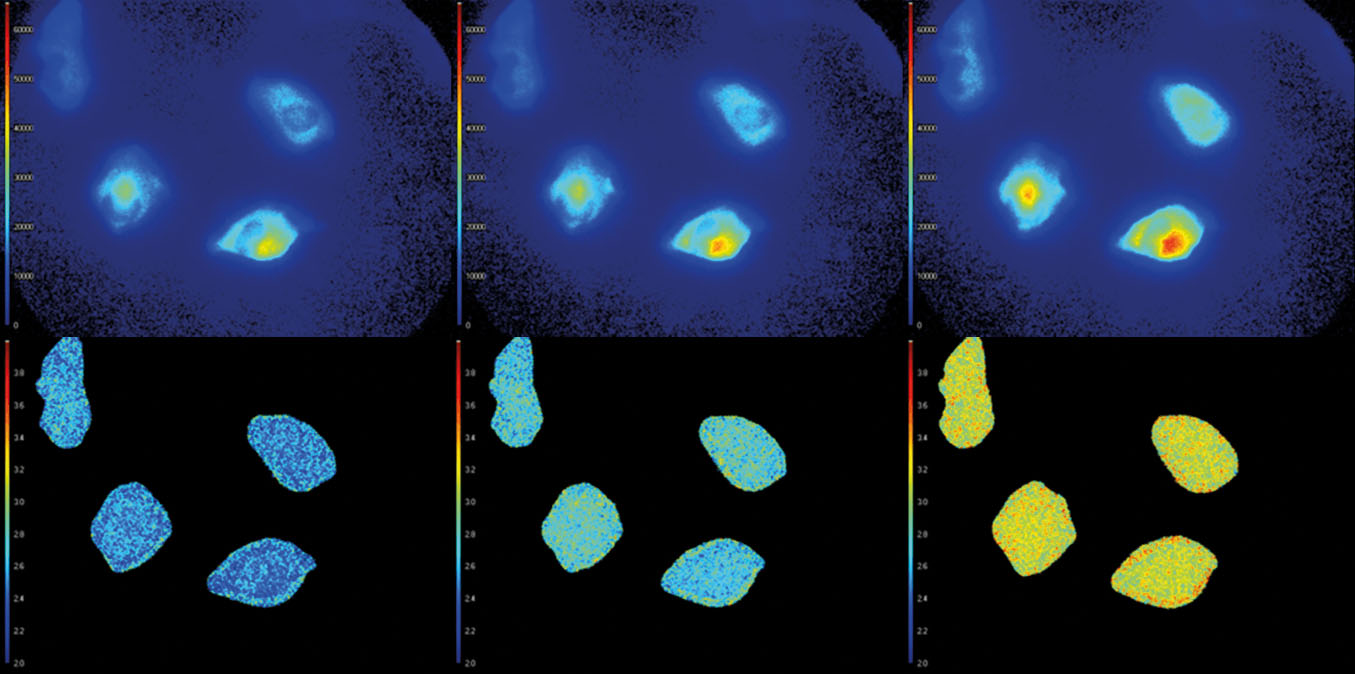
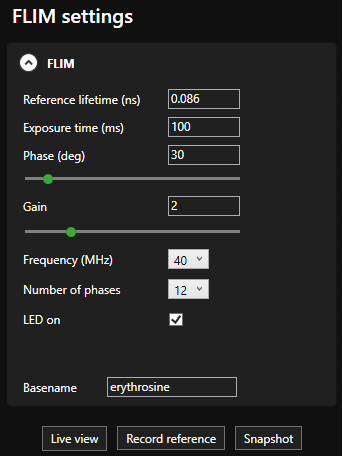
- Automatic data analysis: Dedicated LIFA software instantly calculates the fluorescence lifetime and presents it as a color-coded overlay on the original image.
LIFA system components
- vTau camera: Versatile, plug-and-play FLIM camera for quick and easy setup using ultra-high sensitivity SPAD detector for up to 100 lifetime images per second.
- Light source (Multi-LED / Multi-LASER): Excitation light sources for frequency-domain fluorescence lifetime imaging, supplied according to the wavelengths you require.
- LIFA software: Seamless integration of all hardware for full system control, guiding through FLIM experiments from start to finish, it supports third-party hardware for a flexible and expandable system.
What is Fluorescence Lifetime Imaging Microscopy (FLIM) ?
Fluorescence Lifetime Imaging Microscopy (FLIM) is an advanced imaging technique used in microscopy to study the dynamic properties of fluorophores within biological samples. Unlike traditional fluorescence microscopy, which captures only the intensity of emitted fluorescence light, FLIM measures the time it takes for a fluorophore to return to its ground state after excitation. This information provides insights into the microenvironment of the fluorophores, allowing researchers to distinguish between different molecular species with overlapping fluorescence spectra and study molecular interactions in living cells. FLIM is particularly valuable in areas such as cell biology and medical research, enabling the visualization of subtle changes in cellular processes, the identification of biomolecular interactions, and the detection of cellular abnormalities at the molecular level. This technique has proven instrumental in advancing our understanding of complex biological phenomena and holds great potential for applications in diagnostics and drug development.